Explain the Difference Between Oxidation and Reduction Reactions
When a molecule looses one electron that electron then shifts to the neighboring molecule. Oxidation stands for a loss and.

Difference Between Hydrogenation And Reduction Compare The Difference Between Similar Terms
An oxidation process does not need the presence of oxygen despite its name.
. This is because in reduction a material is gaining an electron while in oxidation the material is losing the electron. Explain process of chemiosmosis. The key difference between oxidation and reduction is that oxidation refers to the loss of electrons while reduction refers to the gain of electrons.
Oxidation does not necessarily have to involve oxygen. C l 2 H 2 2 H C l Reduction of C l 2 to H C l Loss of electron from any species is known as oxidation. A Briefly explain the difference between oxidation and reduction electrochemical reactions.
Gain of hydrogen 3. Reduction is defined as the process when an atom molecule or an ion gains one or more electrons in a chemical reaction. Solution for Briefly explain the difference between oxidation and reduction electrochemical reaction also state which reaction occurs at anode abs which at.
You need to add heat in order to reduce iron ore to produce metallic iron. The reduction reaction involves a gain of electrons and the. Reduction does not necessarily have to involve hydrogen.
Chemical reactions between different compounds are called redox reactions if the oxidation states of reactants are different from those of products. Reduction is the process by which an atom an extra electron for electrons and becomes an anion bOxidation occurs at the reduction at the Q Mu. The oxidation reaction involves a loss of electrons and the substance that has electrons removed from it is said to be oxidized.
5 rows Difference between Oxidation and Reduction. Explain how mitochondrias structure is related to its function. Cro-aq Cth0Hl-- Craq CO2g Question.
Outline process of aerobic respiration including the link reaction kerbs cycle the electron transport chain and role of oxygen. Outline process of glycolysis. Oxidation refers to the loss of electrons by an atom during a chemical reaction.
The one that is loosing electron is oxidation process and the one that is gaining electron is reduction. In oxidation reactions there is a loss of electrons and in reduction reactions there is a gain of electrons. Despite the name oxygen need not be present in an oxidation reaction.
Essentially a reduction reaction occurs when a molecule gains electrons. Redcuction reaction is a reaction in which the atom or molecule gains electron An oxidation reaction is a. An electrochemical reaction involves the transfer of electrons between two chemical compounds.
Gain of electrons Eg. A Explain the difference between oxidation and reduction. It is the process of loss.
9 rows Oxidation reaction. Oxidation occurs when the oxidation state of an atom is increased. Loss of electrons Eg.
Egoroff_w 7 1 year ago. Both oxidation and reductions typically occur at the same time. The electron is typically passed from one material to the other.
M g M g 2 2 e 3. Oxidation S 2 to S The gain of an electron by any species is known as reduction. N a N a e Oxidation of N a to N a S 2 S 2 e.
Reaction in which the molecule looses an electron. Decrease in oxidation number. Vit Answeraoxidation is the process by which an atom an electron for electrons to become a cation.
Loss of hydrogen 2. Reduction Reduction and oxidation occur simultaneously in a type of chemical reaction called a reduction-oxidation or redox. Increase in oxidation number 4.
When reduction occurs the state of the chemical species decreases. The opposite of oxidation is called reduction which occurs when there is a gain of electrons or. The main difference between the reduction and oxidation process is based on gaining and loosing of electron.
Explain difference between oxidation and reduction reactions. Loss of oxygen 2. An active metal electrode was found to lose mass as the oxidation-reduction reaction was allowed to proceed.
A Explain the difference between oxidation and reduction electrochemical reactions. One is loosing electron and other is gaining. Gain of oxygen 1.
A reduction-oxidation or redox reaction is a type of chemical reaction in which reduction and oxidation occur at the same time. The oxidized species loses electrons while the reduced species gains electrons. In a redox reaction the loss of electrons from one substance is called oxidation and the addition of electrons to another substance is known as reduction.
The electrochemistry of oxidation and reduction is very useful in sensors chemical. Redox reaction is short for reduction-oxidation reaction. The addition of hydrogen to a substance is known as reduction.
2 1 C l 2 e C l. B Reduction reaction occur at cathode while oxidation reaction occur at Anode. C Balance the following reactions both in acidic and basic environments.
4 Ratings 19 Votes Answer a Oxidation- Reduction reactions which are commonly termed as Redox reactions are seen in number of organicand inorganic chemical reactions. When iron oxide is reduced to metallic iron it gains oxygen. B Which reaction occurs at the anode and which at the cathode.
Reduction and oxidation both occur simultaneously. Oxidation and reduction reactions are two types of electrochemical reactions. Oxidation never occurs without reduction and reduction never occurs without oxidation.
The main difference between the reduction and oxidation process is based on gaining and loosing of electron. Oxidation-reduction reactions are also known as. The reduced species receives electrons whereas the oxidised species loses them.
Redox is short for reduction-oxidation which is what occurs in any. Was the electrode part of the anode or cathode. Redox reactions are always balanced equations.
The one that is loosing electron is oxidation process and the one that is gaining electron is reduction. An oxidation reaction occurs when a molecule looses electrons. When oxidation occurs the state of the chemical species increases.

Write One Difference Between Oxidation And Reduction Differbetween
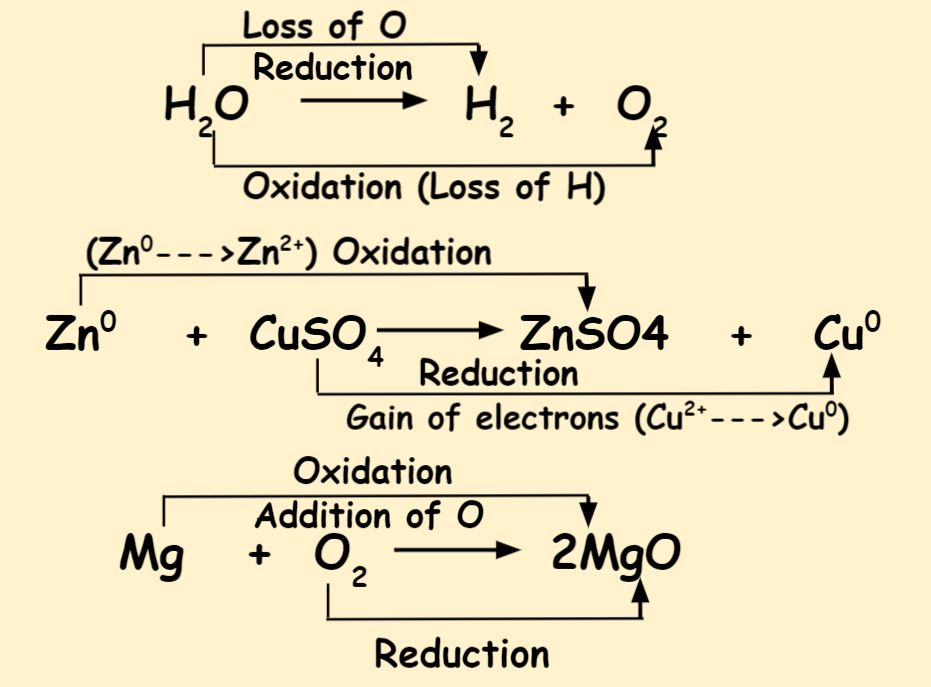
10 Differences Between Oxidation And Reduction Reaction Dewwool

Difference Between Oxidation And Reduction Chemistry Lessons Chemistry Basics Chemistry Classroom
Difference Between Oxidation And Reduction Definition Mechanism Examples
Difference Between Hydrogenation And Reduction Definition Mechanism Examples Relationship And Differences

Difference Between Oxidation And Reduction Redox Reaction Class 11 Electrochemistry 01 Youtube
Oxidation Vs Reduction Definition 8 Major Differences Examples

10 Differences Between Oxidation And Reduction Reaction Dewwool

What Is The Difference Between Oxidation Potential And Reduction Potential Electrode Potential Youtube

Difference Between Oxidation Potential And Reduction Potential Compare The Difference Between Similar Terms

Difference Between Oxidation And Reduction Compare The Difference Between Similar Terms
Difference Between Combustion And Oxidation Reactions Of Ethanol Complete And Incomplete Combustion Oxidation Comparison

Difference Between Oxidation And Reduction Differbetween
Difference Between Oxidation And Reduction Definition Mechanism Examples
Oxidation Vs Reduction How To Remember The Difference Psiberg

Difference Between Oxidation And Reduction Comparison Summary Chemistry Classroom Chemistry Lessons Teaching Chemistry
Difference Between Oxidation Number And Oxidation State Definition Rules Examples

Difference Between Oxidation And Reduction
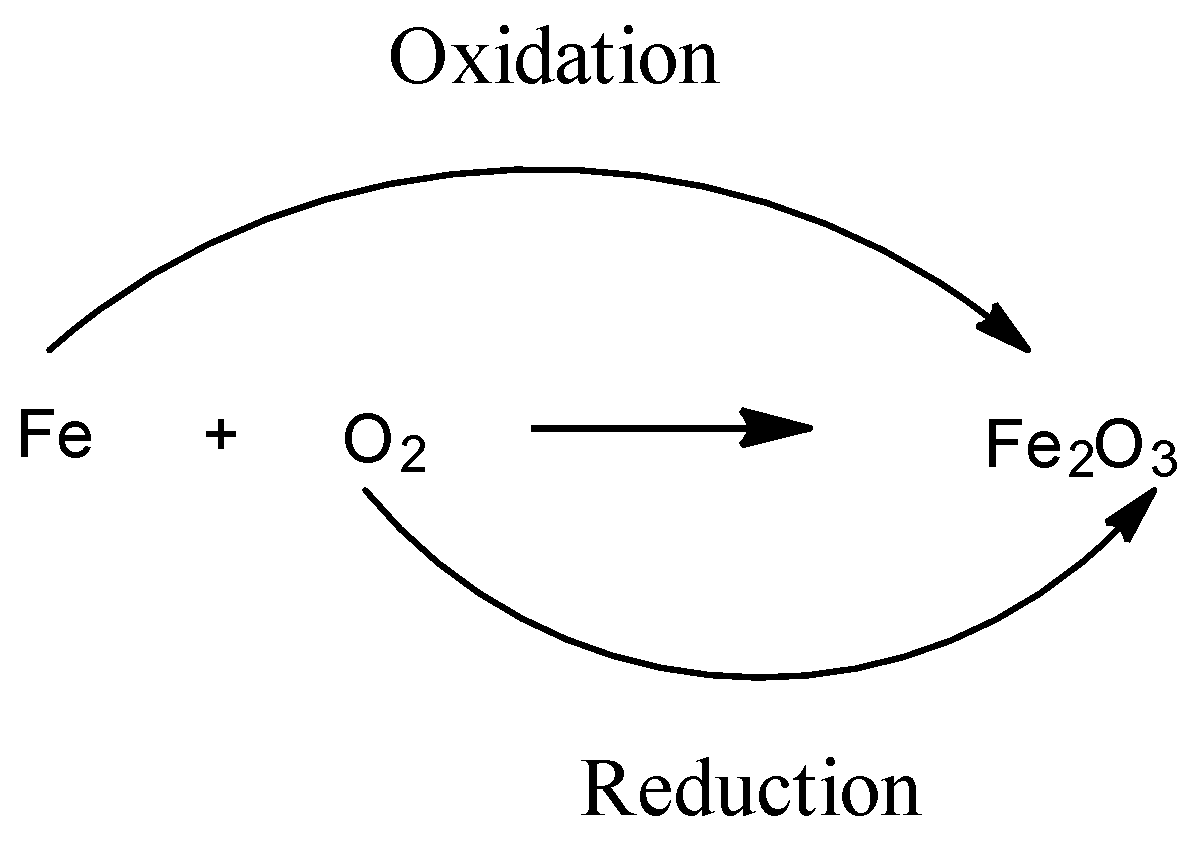
What Is The Difference Between Oxidation And Reduction Class 11 Chemistry Cbse
Comments
Post a Comment